Humidity – not just light – causes color degradation in historical paintings, researchers discover
The discovery will help art conservators develop new preservation techniques.
By David Krause
When you look at a painting in a museum, the colors that you see are likely less bright than they were originally, something that had previously been attributed mainly to light exposure. Now, researchers have discovered a new cause of color degradation: humidity.
“This new degradation pathway explains why some paintings’ colors may fade even when they’ve been stored in dark rooms,” Stanford Synchrotron Radiation Lightsource scientist Sam Webb said. “Many Renaissance-type paints can fade or change color, so understanding what causes these things to happen can help conservationists maintain our masterpieces from the past.”

The research team from SSRL at the Department of Energy’s SLAC National Accelerator Laboratory, the University of Amsterdam, Rijksmuseum and other institutions wanted to explain why a form, or species, of arsenic called As(V) is found alongside a yellow arsenic sulfide pigment called orpiment. Previous research has shown that the conversion of the pigments to As(V) can cause color degradation, but the roots of this change have remained unclear.
To study the As(V) species, the research team took a small sample out of the 17th century painting, “Still Life with Flowers in a Glass Vase,” by Jan Davidszoon de Heem. The yellow eglantine rose at the center of the painting has been fading to white.
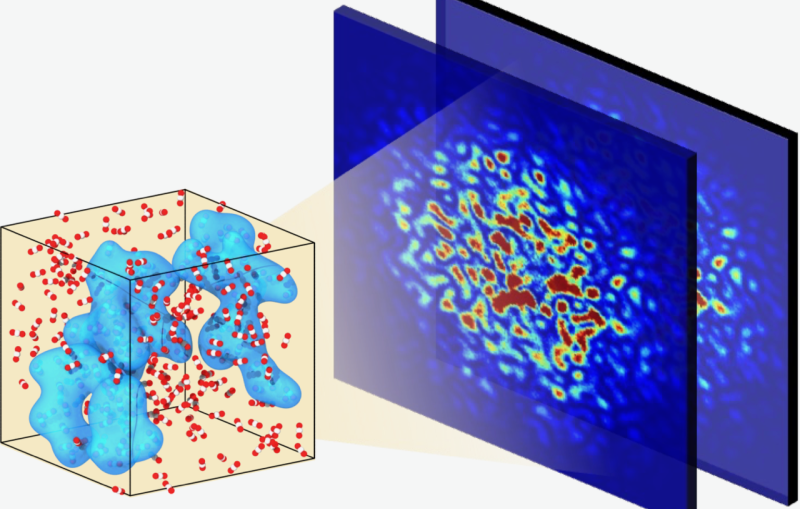
To see the color change in the yellow rose in greater detail, the researchers zapped the sample with bright X-rays generated at one of SSRL’s beam lines. These X-rays provided researchers with 2D images of the sample’s cross section and the locations of the arsenic species.
“SSRL’s X-rays allowed us to get into the depth of the paint,” SSRL scientist Johanna Nelson Weker said. “We saw below the surface layers, which have been exposed to light, and into the layers that haven’t been degraded by light.”
After snapping X-ray images, the researchers placed the paint sample under an X-ray microscope to image the chemistry of the arsenic species in higher resolution and 3D. Combing these X-ray techniques – i.e., X-rays that provided a 2D, birds-eye view of the paint, and X-rays that showed its 3D structure in close detail – allowed the team to map the degradation pathways. These pathways revealed that exposure to humidity creates a trail for the arsenic species to travel on, Nelson Weker said. The researchers published their findings in the Journal of the American Chemical Society.

Additionally, they found that the arsenic species doesn't stay just where the artist used orpiment pigment. Conservators previously thought that degradation was concentrated at where the pigment was in a painting.
“This means that if you are a conservator, you have to not only look at where the arsenic pigment is, but in a large halo around the pigment,” Webb said.
Discovering that humidity could help degrade colors took a lot of trial and error, Webb said. First, the team recreated a sample of the arsenic pigment from scratch in the lab. Then they added egg yolk – a common pigment binder used by artists of the time – to the sample. They tested this egg-yolk sample under different humidity conditions in the lab and then compared their results with what they saw within the sample from de Heem’s yellow eglantine rose. That revealed that the arsenic species can spread after reacting with water in lab-grown samples and in paintings that are hundreds of years old.
In the future, the team wants to understand in greater detail the interactions between light, including from experimental X-rays, and the pigments. This will help them understand all of the reactions between the pigments and the binders in a more complex setting and further help conservation efforts, Webb said. Additionally, the researchers would like to determine the precise range of humidity levels that allow the arsenic species to grow in the pigment.
SSRL is a DOE Office of Science user facility.
Citation: T. H. Broers, Webb, Nelson Weker, et al., Journal of The American Chemical Society 14 April 2023 (doi/10.1021/jacs.2c12271)
Contact
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
About SLAC
SLAC National Accelerator Laboratory explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by researchers around the globe. As world leaders in ultrafast science and bold explorers of the physics of the universe, we forge new ground in understanding our origins and building a healthier and more sustainable future. Our discovery and innovation help develop new materials and chemical processes and open unprecedented views of the cosmos and life’s most delicate machinery. Building on more than 60 years of visionary research, we help shape the future by advancing areas such as quantum technology, scientific computing and the development of next-generation accelerators.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.